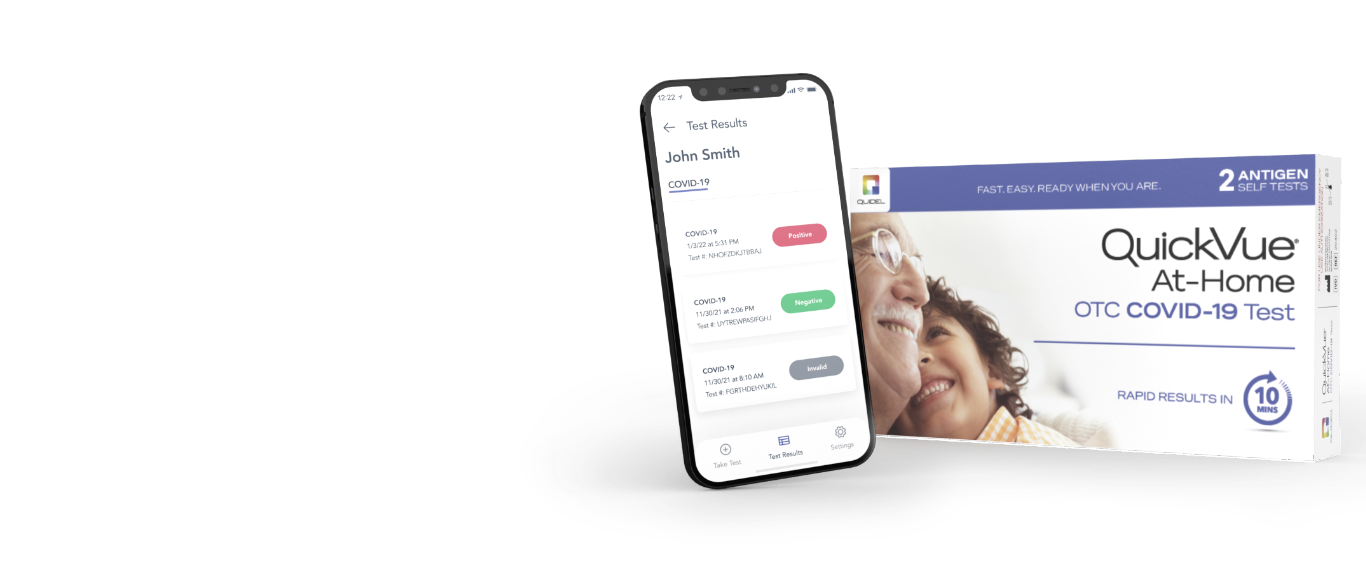
QuickVue At Home
This is a carousel with auto-rotating slides. Use play/pause button to disable auto rotation. You can jump to a slide with the slide dots at the bottom.
Fast. Easy. Ready when you are.
Effectively detects Omicron and other variants
Latest updates
Swab, swirl, dip, and see results
QuickVue At-Home OTC COVID-19 Test is fast, simple, and easy-to-use designed with at-home users in mind. The test uses a gentle nasal swab sample to determine a positive or negative COVID-19 test result. The swab is swirled in a tube of reagent solution, then removed. A test strip is then inserted into the solution. After 10 minutes, the test strip is removed from the tube to confirm results.
Before initiating the test, it is important to first read and closely follow the detailed instructions included in the package. Watch our test demonstration video for a quick overview.
Swab, swirl, dip, and see results
QuickVue At-Home OTC COVID-19 Test is fast, simple, and easy-to-use designed with at-home users in mind. The test uses a gentle nasal swab sample to determine a positive or negative COVID-19 test result. The swab is swirled in a tube of reagent solution, then removed. A test strip is then inserted into the solution. After 10 minutes, the test strip is removed from the tube to confirm results.
Before initiating the test, it is important to first read and closely follow the detailed instructions included in the package. Watch our test demonstration video for a quick overview.
- Gently swirl the nasal swab 4 times in each nostril.Gently swirl the nasal swab 4 times in each nostril.
- Place the nasal swab in the solution tube, twist 3-4 times, and remove the swab.Place the nasal swab in the solution tube, twist 3-4 times, and remove the swab.
- Place the test strip into the tube.Place the test strip into the tube.
- Easy-to-read results are available in just 10 minutes.Easy-to-read results are available in just 10 minutes.

Click a retailer below to shop now or find a store near you
You are about to leave the QuidelOrtho website for a third-party site. Links which take you outside of the QuidelOrtho website are not under QuidelOrtho’s control, and QuidelOrtho is not responsible for any content or links contained therein. QuidelOrtho is providing these links to you only as a convenience, and such provision does not imply an endorsement by QuidelOrtho of any linked site. Any information you provide to a third-party site will be governed by the third-party’s website Terms of Use, including those related to confidentiality, data privacy and security.