COVID-19 Resources
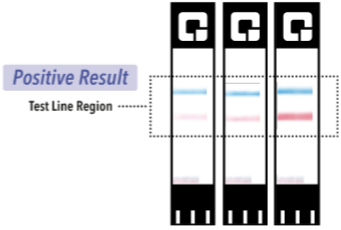
What happens if I receive a positive test result?
If you test positive with the QuickVue At-Home OTC COVID-19 Test, then proteins from the virus that causes COVID-19 has been found in your sample and you likely have COVID-19.
Per CDC recommendations, you should self-isolate at home to help stop the spread of the virus to others. For the latest CDC recommendations and updates, visit the CDC website »
The QuickVue At-Home OTC COVID-19 Test expiration extension
The QuickVue At-Home OTC COVID-19 Test has been granted FDA EUA authorization to extend expiration dating from 12 months to 16 months. A copy of the letter is available here. Please note the expiration dates on the outer kit box may not reflect the 16-month dating for product that is already distributed.
Is your test nearing its expiration date?
To verify if the expiration date for a particular lot has been extended:
- Go to the FDA webpage »
- Scroll down to view the list of lot numbers with updated expiration dating. You will require the lot number on your test kit box.

Information regarding
reimbursement of
COVID Tests
Documents and resources
You are about to leave the QuidelOrtho website for a third-party site. Links which take you outside of the QuidelOrtho website are not under QuidelOrtho’s control, and QuidelOrtho is not responsible for any content or links contained therein. QuidelOrtho is providing these links to you only as a convenience, and such provision does not imply an endorsement by QuidelOrtho of any linked site. Any information you provide to a third-party site will be governed by the third-party’s website Terms of Use, including those related to confidentiality, data privacy and security.