Frequently Asked Questions
The QuickVue At-Home OTC COVID-19 Test is a type of test called a rapid antigen test. Antigen tests are designed to detect proteins from the virus that causes COVID-19, in anterior nasal swabs.
For a demonstration on how the test works, watch the instructional video:
Step 1. Gently swirl nasal swab four times in each nostril
Step 2. Place nasal swab in the solution tube, twist 3-4 times, and remove the swab
Step 3. Place test strip into the tube
Step 4. Easy-to-read results are available in just 10 minutes
To find out if your test kit’s expiration date has been extended and to verify the new expiration date, please select the link below and search for your kit’s lot number. The updated expiration date will be listed in the “Now have this extended expiration date” column. If your lot number is not found on this list, it has not been date-extended, and the expiration date listed on the kit box should be used. Link: https://www.fda.gov/media/162056/download
The QuickVue At-Home OTC COVID-19 Test has been granted FDA EUA authorization to extend expiration dating from 12 months to 16 months. A copy of the letter is available here. Please note the expiration dates on the outer kit box may not reflect the 16-month dating for product that is already distributed.
To verify if the expiration date for a particular lot has been extended:

QuidelOrtho does not recommend using expired kits.
The expiration date is dependent upon different factors. Although the lot numbers are similar, the expiration date of the components within the kit may be different and may impact if the kit is extended.
Clinical studies have shown that antigen tests more accurately determine whether you are infected with the virus that causes COVID-19 when taken multiple times across several days. Repeat testing improves test accuracy. This serial testing approach is recommended to minimize the risk of incorrect results. For more information on the performance of the test and how the performance may apply to you, please refer to the performance data in the Healthcare Provider Instructions for Use (IFU).
Results are available in as little as 10 minutes in the privacy of your own home.
At QuidelOrtho, we continuously monitor the evolution and activity of COVID-19 variants in circulation and will continue to be vigilant in evaluating our tests with real-world virus samples to assure you of our product’s efficacy. QuidelOrtho has completed testing on several variant strains and the test was able to detect the mutations. Because the test detects a part of the virus that is less susceptible to mutation, the likelihood of detecting new or emerging variants is high. QuidelOrtho monitors the variants closely and will inform the FDA promptly, should any issues be detected.
The type of testing and documentation required for air travel may differ based on travel destination, airline, and state/country requirements. We encourage you to visit the CDC/TSA website as well as the airport, airline, and health department’s website for the latest requirements on the type of acceptable testing and documentation for your travel destination.
There are different kinds of tests for diagnosing COVID-19. Molecular tests (also known as PCR tests) detect genetic material from the virus. Antigen tests detect proteins from the virus. Antigen tests are very specific for the virus but are not as sensitive as molecular tests. This means that a positive result is highly accurate, but a negative result does not rule out infection.
There are different kinds of tests for the SARS-CoV-2 virus that causes COVID-19. Molecular tests detect genetic material from the virus. Antigen tests, such as the QuickVue COVID-19 Test, detect proteins from the virus. Due to the lower sensitivity of antigen tests, there is a higher chance this test will give you a false negative result when you have COVID-19 than a molecular test would.
The solution in the tube contains small amounts of hazardous ingredients (see table on page 5 of the User Instructions here). If the solution contacts the skin or eye, flush with plenty of water. If irritation persists, seek medical advice at https://www.poison.org/contact-us or 1.800.222.1222.
COVID-19 serial testing is when one person tests themselves multiple times for COVID-19 on a routine basis, such as every day or every other day. By testing more frequently, you may detect COVID-19 more quickly and reduce spread of infection. Serial testing (i.e., testing every day or every other day) is more likely to detect COVID-19, especially when you do not have any symptoms.
This test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older.
No, the nasal swab is not sharp, and it should not hurt. Sometimes the swab can feel slightly uncomfortable. If you feel pain, please stop the test and seek advice from a healthcare provider.
The test is intended for the individuals with or without symptoms or other epidemiological reasons to suspect COVID-19.
Potential risks include:
- Possible discomfort during sample collection.
- Possible incorrect test results (see Results section).
Potential benefits include:
- The results, along with other information, can help your healthcare provider make informed recommendations about your care.
- The results of this test may help limit the spread of COVID-19 to your family and others in your community.
The United States FDA has made this test available under an emergency access mechanism called an Emergency Use Authorization (EUA). The EUA is supported by the Secretary of Health and Human Service’s (HHS’s) declaration that circumstances exist to justify the emergency use of diagnostics (IVDs) for the detection and/or diagnosis of the virus that causes COVID-19. An IVD made available under an EUA has not undergone the same type of review as an FDA-approved or cleared IVD. FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, available alternatives, and based on the totality of scientific evidence available, it is reasonable to believe that this IVD may be effective in diagnosing COVID-19. The EUA for this test is in effect for the duration of the COVID-19 declaration justifying emergency use of IVDs, unless terminated or revoked (after which the test may no longer be used).
The test kit comes with two tests intended to be used for the same patient. A 25-test kit is also available.
Individuals with or without symptoms can still utilize this test, as needed, regardless of vaccination status.
Serial testing should be performed in individuals with negative results at least twice over three days (with 48 hours between tests) for symptomatic individuals and three times over five days (with at least 48 hours between tests) for asymptomatic individuals. You may need to purchase additional tests to perform this serial (repeat) testing.
You do not need a doctor’s prescription to purchase and perform this test.
Please visit the Say Yes! COVID Test website for more information. The FAQ can be found here.
No, your iPhone or Android camera app is sufficient. Point the camera at the QR code and tap the notification link to proceed to the website.
No, the QuickVue At-Home OTC COVID-19 Test kit does not produce a QR code that can be scanned and verified by a third party. However, if you download QVue, the companion app to the QuickVue COVID-19 Test, you can use the app to perform the test and receive a digital result record that is shareable with whomever you want, whenever you want. Please open your iPhone App Store or Android Play Store and search for QVue.
Yes, QVue, the companion app to the QuickVue At-Home OTC COVID-19 Test, provides access to shareable digital results records, in addition to simple-to-follow animated instructions, built-in timers, and assisted result interpretation.
Only a pink line about a half of a centimeter below the blue control line in the Test Line Region should be considered a positive result. Pink lines in any other are of the test strip should not be called a positive result.
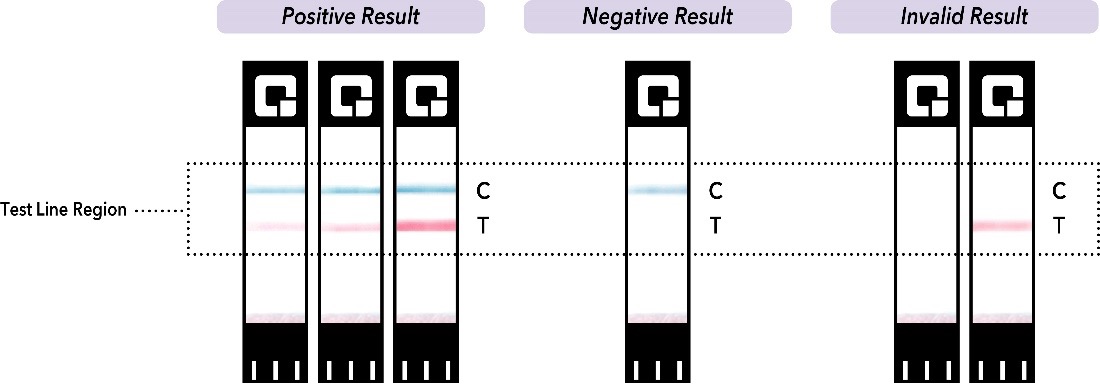
Only a pink line about half of a centimeter below the blue control line should be considered a positive result. A pink line bordering the black label with the arrows, a vertical pink line, or a faint grey line next to the blue control line is not considered a positive test line and should not be called a positive result.

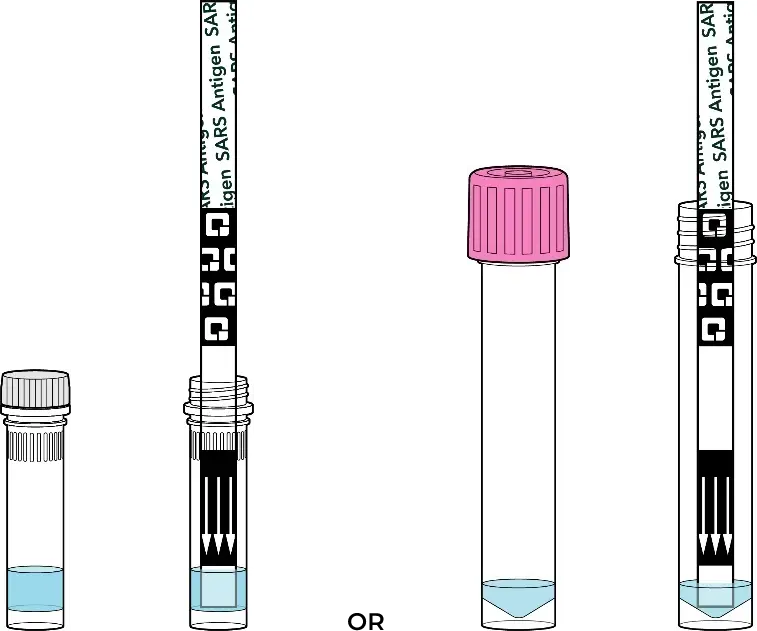
The tube contains a small amount of liquid that only fills the bottom, as illustrated below. The amount of liquid may not look exactly like the photo in the User Instructions. The liquid in the tube should cover the bottom of the swab and test strip when immersed. The entire swab tip and arrows on the test strip do not need to be completely covered by the liquid. Please ensure to stir the swab 3-4 times in the liquid before the 1-minute incubation step.
Examples of tubes and expected liquid solution levels:
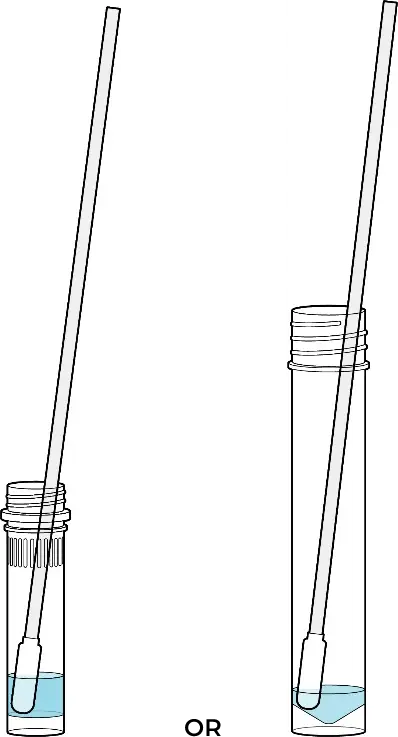
The amount of liquid should cover approximately ¾ of the swab when it is immersed. It is important to follow the User Instructions and stir the swab 3-4 times once immersed in the tube. After the 1-minute incubation in the solution, remove the swab from the TUBE by rubbing the swab head against the inside wall of the tube to squeeze out as much liquid as possible.
The QuickVue At-Home OTC COVID-19 Test should always be stored upon receipt according to the temperature printed on the kit box (59°F to 86°F or 15°C to 30°C). QuidelOrtho has performed studies that demonstrate the product performs as expected under different temperature conditions (i.e., heated, and frozen conditions) encountered during shipping. If you have any concerns about the shipping conditions of your test kit, please contact the retailer where you purchased the test.
The FDA also posted guidance to consumers on their website here.
The expiration date is labeled on the outside of the kit box, under the lot number, next to the hourglass symbol. There is also a date printed next to the symbol of a building with a chimney and this is the date of manufacture.

The QuickVue At-Home OTC COVID-19 Test should always be stored upon receipt according to the temperature printed on the kit box (59°F to 86°F or 15°C to 30°C).
The kit components may look slightly different than the User Instructions. You may observe the following kit differences:
- A different sized tube
- A different color cap on the tube (e.g., an orange or purple cap)
- Different brands of swabs
- A different tray and tube holder
- A white or clear tray
The test is only authorized for use with nasal swab specimens, and the accuracy and performance of throat swab specimens have not been evaluated. Throat swabs are not recommended and may not produce accurate results.
The test is intended to be read only at 10 minutes. If the test is read more than 5 minutes after the indicated read time, the result may be inaccurate and should not be used.
QuidelOrtho recently updated the kit instructions to clarify the best timeframe to use the test. Please refer to the User Instructions for the most up-to-date instructions.
No. Each test is designed for single use by one individual. We recommend keeping the User Instructions and tube holder until the second test is completed.
You can download the QVue app by searching and selecting “QVue” in the App Store® or Google Play™, or by scanning the QR code on the outside panel of the box. Learn more on our dedicated QVue page.
The QVue app is not required to take your QuickVue At-Home OTC COVID-19 Test, but it can make your testing experience less stressful and increase confidence in your result. The app provides clear, visual step-by-step instructions from opening your test through result interpretation, taking the guesswork out of testing, and helping ensure your test and results are valid. By completing your test with the QVue app, you can easily access a history of results for you and your family from a single place and share a record of your result with whomever, whenever you want. For new and experienced testers, the QVue app provides an easy and stress-free way to test for COVID-19.
QVue makes it convenient and super easy to share a record of your result to whomever, whenever you want. Select the “Test Result” tab on the menu, choose the user, and then select the specific result from your history that you want to share. It’s as easy as sharing a photo with a friend. Select “Share,” then choose who you want to send it to and how—text, e-mail, or another app enabled on your phone.
No, we are not selling your data. Protecting your private health information is our priority, and we do not sell, rent, or lease user information to third parties. Read more about our privacy policy here.
Per CDC guidelines, specific federal and state regulations mandate reporting test results to appropriate public health agencies. The personal information collected in QVue is strictly used to comply with these guidelines. We do not sell or distribute your data without your consent, as privacy is of utmost importance to us. All data is encrypted and not shared with any other third parties.
While taking the test and following the guided instructions, we recommend you stay in the app until the test is complete. Timing is critical to ensure a valid test result. You can open other apps or browse the web, but the app must remain open in the background. If you force close the app, you will have to start the testing process over and use new test components. We suggest keeping your phone sound volume on so that you receive an audible notification from the app when critical timing steps are complete.
Once the 10-minute development time is complete, you have an additional 5-minutes to read your results. Timing is essential to receiving a valid and reliable test result, so it is important to read your result as soon as possible. If you do not read and input the result before the end of the additional 5 minutes, you will receive a notification that your test has expired, and your result is invalid.
Timing is critical to ensure you receive a valid and reliable test result. If you did not complete the steps to read and input your result into the app within the 5 minute countdown window, you receive an expired test notification, and the result is recorded as invalid. The stability of the result is not reliable after 5 minutes. Therefore, we enforce that the result is read and recorded during this time to ensure we provide you with the most reliable result reading. Please perform a new test with a new nasal swab sample and test strip and be sure to read your result within the 5 minute window.
The type of testing and documentation required for air travel may differ based on the travel destination, airline, and state/country requirements. We encourage you to visit the CDC/TSA website and the airport, airline, and health departments’ websites for the latest requirements for testing and supporting documentation required for your travel destination.
The type of testing and accompanying documentation required for your workplace or event may differ. As a result, we recommend you review your workplace/event requirements to ensure you provide the correct documentation.
First, make sure that you have the latest version of the app installed. If the issue persists, try uninstalling the QVue app and reinstalling it. Please get in touch with Technical Support if you continue to experience problems on our Contact Us page.
QVue, the companion app to the QuickVue At-Home OTC COVID-19 Test, provides access to sharable digital results records. Please visit the website for your airline or airport to view their latest requirements on the type of documentation required for your travel destination.
Download QVue, the companion app to the QuickVue At-Home OTC COVID-19 Test, and use the app to perform the test and receive a digital result record that is shareable with whomever you want, whenever you want. Please open your iPhone App Store or Android Play Store and search for QVue.
Individuals who test positive with the QuickVue At-Home OTC COVID-19 Test should self-isolate and seek follow-up care with their physician or healthcare provider as additional testing may be necessary.
If you test positive with the QuickVue At-Home COVID-19 Test you should self-isolate and seek follow-up care with your healthcare provider as additional testing may be necessary. Your healthcare provider will work with you to determine how best to care for you based on your test result(s) along with your medical history and your symptoms
A positive test result means that it is very likely you have COVID-19 because proteins from the virus that causes COVID-19 were found in your sample. You should self isolate from others and contact a healthcare provider for medical advice about your positive result.
A negative test result indicates that antigens from the virus that causes COVID-19 were not detected in your sample. However, if you have symptoms of COVID-19, and your first test is negative, you should test again in 48 hours since antigen tests are not as sensitive as molecular tests. If you do not have symptoms and received a negative result, you should test at least two more times with 48 hours in between tests for a total of three tests. If you have a negative result, it does not rule out SARS-CoV-2 infection; you may still be infected and you may still infect others. It is important that you work with your healthcare provider to help you understand the next steps you should take.
An invalid result means the test was not able to tell if you have COVID-19 or not. If the test is invalid, a new swab should be used to collect a new nasal specimen and you should test again with a new test.
Please follow the latest CDC guidelines and communicate your results to your healthcare provider. Healthcare providers are responsible for reporting COVID-19 test results to the appropriate authorities.
Coverage varies based on the health insurance plan. Please check with your plan for details.
After the end of the PHE in May 2023, mandatory coverage for over-the-counter and laboratory-based COVID-19 tests ended. Coverage will vary depending on the health insurance plan. Details regarding coverage can be found in the following links:





