Mobile Solutions
Take your test, lose the stress
QVue empowers you to do more with your QuickVue Test
From testing to timing to tracking, animated instructions guide you through the testing process and improve your confidence in your results. Access your test results so you can save and share them with whomever you want, whenever you want.
QVue is designed to support the entire QuickVue At-Home Test portfolio. Currently, QVue offers support for the QuickVue At-Home OTC COVID-19 Test.*
*For use under FDA Emergency Use Authorization (EUA) only.

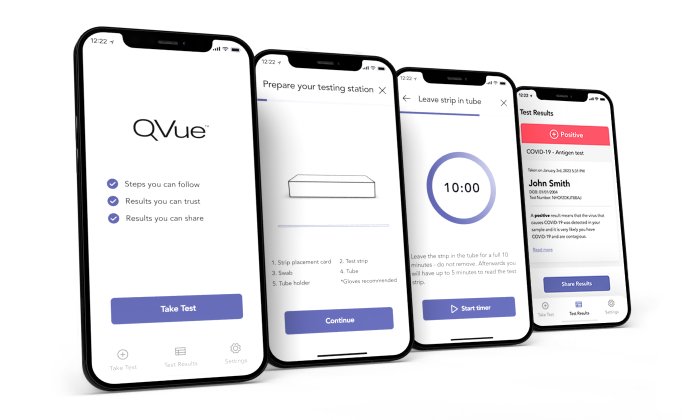
QVue has you covered
-

Test without stress
With animated steps to help you through the process
-

Share results
With whomever you want, whenever you want, directly from the app
-

Access results
Save test results for you and your family so you can access and share when needed
Change the way you test
-
Setup is simple
Create an account and start testing within a few taps
-
Keep everything in one place
Conveniently save and access test results for you and your family
-
Collect with confidence
Step-by-step guidance from opening your test kit to
reading results -
Quick solutions for everything testing
Easily share a digital record of your results with whomever you want, whenever you want

Test with confidence
Google Play and the Google Play Logo are trademarks of Google LLC.
QVue general FAQs
How do I download the QVue app?
You can download the QVue app by searching and selecting “QVue” in the App Store® or Google Play™, or by scanning the QR code on the outside panel of the box. Available on Apple iPhones with iOS 13 or higher and Android smartphones with Android 9 or higher.
Do I need to use the QVue app to take the test?
The QVue app is not required to take your QuickVue® At-Home OTC COVID-19 Test,* but it can make your testing experience less stressful and increase confidence in your result. The app provides clear, visual step-by-step instructions from opening your test through result interpretation, taking the guesswork out of testing and helping ensure your test and results are valid.
By completing your test with the QVue app, you can easily access a history of results for you and your family from a single place and share a record of your result with whomever, whenever you want. For new and experienced testers, the QVue app provides an easy and stress-free way to test for COVID-19.
How can I share my test results from QVue?
QVue makes it convenient and super easy to share a record of your result to whomever, whenever you want. Select the “Test Result” tab on the menu, choose the user, and then select the specific result from your history that you want to share. It’s as easy as sharing a photo with a friend. Select “Share,” then choose who you want to send it to and how—text, e-mail, or another app enabled on your phone.